
(iii) Seema takes a blue crystalline salt P in a test tube. (b) Write a balanced chemical equation for the above reaction. To demonstrate this, she passes ammonia gas over heated copper oxide. (i) (a) Ranjana wants to prove that ammonia is a reducing agent. (c) Balanced equation for the reaction occurring (iv) With respect to Haber’s process answer the following: (a) Action of warm water on Aluminium nitride. (iii) Give balanced equations for each of the following: Find the relative molecular mass of the gas. When the same cylinder is filled with hydrogen gas at the same temperature and pressure the mass of hydrogen is 2 g. (ii) A gas cylinder of capacity 40 dm3 is filled with gas X the mass of which is 20 g. (b) Give a balanced equation for the reaction that takes place at the cathode. (a) Name the other aluminum containing compound added to alumina. (i) The following questions relate to the extraction of Aluminium by electrolysis. (b) Calculate the number of moles in 22 grams of carbon dioxide. The compound PQ2 is a good conductor of electricity. In the formation of a compound PQ2, atom P gives one electron to each atom of Q. In an electrovalent compound, the cation attains the electronic configuration of the noble gas that comes after it in the periodic table.Ģ. (iv) (a) State wether the following statements are TRUE or FALSE.

(c) State whether X is likely to be placed to the left or to the right of Y in the periodic table? (b) How is the electronegativity of X likely to compare with that of Y? (a) How is the oxidising power of X likely to compare with that of Y? (iii) The electron affinity of an element X is greater than that of element Y. Sulphuric acid reacts with Copper to produce Sulphur dioxide gas. (a) In the preparation of HCl gas when it reacts with Sodium chloride. (ii) What property of Sulphuric acid is exhibited in each of the following cases: Nitric acid reacts with compound Q to give a salt Ca(NO3)2, water and carbon dioxide. (i) Identify the reactant and write the balanced equation for the following: The intended marks for questions or parts of questions are given in brackets SECTION B Chemistry ICSE Specimen Paper Solved 2024 Class-10 Attempt any four questions from Section B. The time given at the head of this Paper is the time allowed for writing the answers.This time is to be spent in reading the question paper.You will not be allowed to write during first 15 minutes.Time allowed: Two and half hours Answers to this Paper must be written on the paper provided separately.If the symptoms continue after a consultation with your doctor, immediately go to the hospital. If you happen to swallow some barium nitrate, please see a doctor immediately.
#Barium nitrate precipitate skin#
While skin or eye contact is less harmful than ingestion or inhalation, it can still result in irritation, itching, redness, and pain. Inhalation may also cause irritation to the respiratory tract. Solutions of sulfate salts such as Epsom salts or sodium sulfate may be given as first aid for barium poisoning, as they precipitate the barium as the insoluble (and non-toxic) barium sulfate. Barium nitrate may also cause kidney damage. Death may result from cardiac or respiratory failure, and usually occurs a few hours to a few days following exposure to the compound. Symptoms of poisoning include tightness of muscles (especially in the face and neck), vomiting, diarrhea, abdominal pain, muscular tremors, anxiety, weakness, labored breathing, cardiac irregularity, and convulsions. Like all soluble barium compounds, barium nitrate is toxic by ingestion or inhalation.

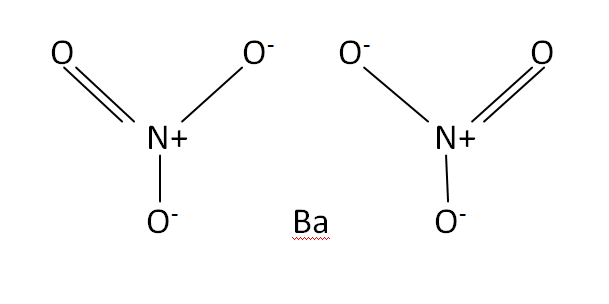
The second requires combining barium chloride with a heated solution of sodium nitrate, causing barium nitrate crystals to separate from the mixture. The first involves dissolving small chunks of barium carbonate in nitric acid, allowing any iron impurities to precipitate, then filtered, evaporated, and crystallized. It is also used in the manufacturing process of Barium oxide, the vacuum tube industry and for green fire in pyrotechnics.īarium nitrate is manufactured by one of two processes. It is mixed with Thermite to form Thermate-TH3, used in military thermite grenades. Barium nitrate mixed with aluminum powder, a formula for flash powder, is highly explosive. It is soluble in water, and like other soluble barium compounds, is toxic and should be handled with care.īaratol is an explosive composed of barium nitrate, TNT and binder the high density of barium nitrate results in baratol being quite dense as well. Template:Chembox new Barium nitrate with chemical formula Ba( N O 3) 2 is a salt of barium and the nitrate ion.īarium nitrate exists as a white solid at room temperature.


 0 kommentar(er)
0 kommentar(er)
